Answer: - the velocity for the forward reaction equal that of the reverse reaction
Explanation:-
Equilibrium constant is defined as the ratio of concentration of product to the concentration of reactants each raised to the power their stoichiometric ratios. It is represented by the symbol 'K'. For the general equilibrium equation:
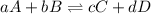
The expression for equilibrium constant is given as:
![K=([C]^c[D]^d)/([A]^a[B]^b)](https://img.qammunity.org/2020/formulas/chemistry/high-school/tqxrmb6bnlx043v6ru23ck9c0d0oa93yyi.png)
Characteristics of equilibrium reaction:
Chemical equilibrium are attained is closed system.
The macroscopic remains constant like: volume, pressure, energy etc.
Rate of forward reaction is equal to the rate of backward reaction. The concentration of the reactants and products remain constant.They are not always equal.