Answer: Option (D) is the correct answer.
Step-by-step explanation:
The acetic acid,
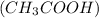 present in vinegar reacts wit magnesium and therefore, it releases hydrogen gas and magnesium acetate.
present in vinegar reacts wit magnesium and therefore, it releases hydrogen gas and magnesium acetate.
The chemical reaction will be as follows.
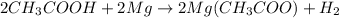
An acid is a substance which has pH less than 7 and changes blue litmus red. Acids taste sour as they are acidic in nature and releases hydrogen ions.
Whereas bases have pH greater than 7 and changes red litmus into blue.Bases taste bitter and releases hydroxide ions.
Thus, we can conclude that the statement it reacts with Mg to produce
 best describes the acid found in vinegar (acetic acid).
best describes the acid found in vinegar (acetic acid).