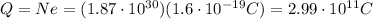Answer:
Step-by-step explanation:
The mass of one electron is
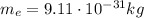
So the number of electrons contained in M=1.7 kg of mass is
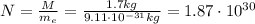
The charge of one electron is
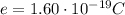
So, the total charge of these electrons is equal to the charge of one electron times the number of electrons: