Answer:
|F| = 2.09 × 10⁻⁸ assuming that the two ions are point charges.
Step-by-step explanation:
What's the charge on each ion?
The symbol
 here stands for fundamental charge. Each electron carries a negative fundamental charge of -e. Each proton carry a positive fundamental charge of +e.
here stands for fundamental charge. Each electron carries a negative fundamental charge of -e. Each proton carry a positive fundamental charge of +e.
Molecules and atoms are neutral. They contain an equal number of electrons and protons. Remove one electron from a molecule or atom, and that particle will end up with more protons (which are positive) than electrons. That particle will carry a positive charge of +e become an ion (a cation to be precise.) Remove another electron and the ion will carry a charge of +2e.
For each ion
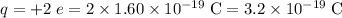 .
.
What's the size of the electrostatic force between the two ions?
Consider Coulomb's Law for the electrostatic force
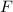 between two point charges:
between two point charges:
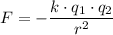 ,
,
where
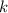 is Coulomb's constant,
is Coulomb's constant,
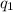 and
and
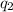 are the charge on the two point charges, and
are the charge on the two point charges, and
 is the separation between the two charges.
is the separation between the two charges.
Make sure that all values are in SI units. Assume that the two ions are small enough that they act like point charges:
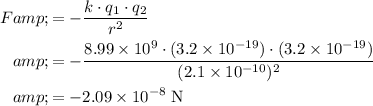 .
.
The value of
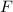 is negative, meaning that the two charges will repel each other because they are both positive. The question is asking for the magnitude of this force. Thus drop the sign in front of
is negative, meaning that the two charges will repel each other because they are both positive. The question is asking for the magnitude of this force. Thus drop the sign in front of
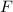 to obtain
to obtain
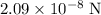 , which is the magnitude of
, which is the magnitude of
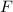 .
.