Answer: The substance which gets reduced in the hydrogen-oxygen fuel cell is oxygen.
Step-by-step explanation:
A reduced substance undergoes reduction reaction. This is defined as the reaction in which an atom gains electrons.
A fuel cell is defined as the electrochemical cell which converts the chemical energy of a fuel (often used hydrogen) and an oxidizing agent (often used oxygen) into electrical energy via a pair of redox reactions.
The reactions which occur in hydrogen-oxygen fuel cell are:
At cathode:
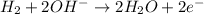
At anode:
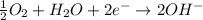
As, the oxygen is gaining electrons from the above reaction. Thus, it is undergoing reduction reaction and is getting reduced.
Hence, the substance which gets reduced in the hydrogen-oxygen fuel cell is oxygen.