Answer:
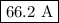
Step-by-step explanation:
1. Write the equation for the reaction.
M_r: 24.30
MgCl₂ ⟶ Mg + Cl₂
m/g: 60.0
2. Calculate the moles of Mg
Moles of Mg = 60.0 g Mg × (1 mol Mg/ 24.30 g Mg) = 2.469 mol Mg
3. Calculate the moles of electrons
Moles of electrons = 2.469 mol Mg × (2 mol electrons/1 mol Mg)
= 4.938 mol electrons
4. Calculate the number of coulombs
Q = 4.938 mol electrons × (96 485 C/1 mol electrons) = 476 500 C
5. Calculate the current required
Q = It
I = Q/t
t = 2.00 h × (60 min/1h) × (60 s/1 min) = 7200 s
I = 476 500 C/7600 s= 66.2 C/s = 66.2 A
You need a current of
 .
.