Answer:
0.21 M. (2 sig. fig.)
Step-by-step explanation:
The molarity of a solution is the number of moles of the solute in each liter of the solution. The unit for molarity is M. One M equals to one mole per liter.
How many moles of NaOH in the original solution?
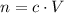 ,
,
where
 is the number of moles of the solute in the solution.
is the number of moles of the solute in the solution.
 is the concentration of the solution.
is the concentration of the solution.
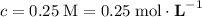 for the initial solution.
for the initial solution.
 is the volume of the solution. For the initial solution,
is the volume of the solution. For the initial solution,
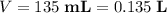 for the initial solution.
for the initial solution.
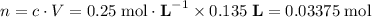 .
.
What's the concentration of the diluted solution?
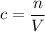 .
.
 is the number of solute in the solution. Diluting the solution does not influence the value of
is the number of solute in the solution. Diluting the solution does not influence the value of
 .
.
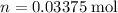 for the diluted solution.
for the diluted solution.- Volume of the diluted solution:
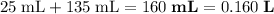 .
.
Concentration of the diluted solution:
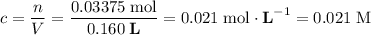 .
.
The least significant number in the question comes with 2 sig. fig. Keep more sig. fig. than that in calculations but round the final result to 2 sig. fig. Hence the result: 0.021 M.