Hello!
The answer is:
The approximate volume is 9.84 L.
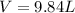
Why?
Since we are given the numer of moles, the temperature and the pressure of the gas, we can calculate the approximate volume using The Ideal Gas Law.
The Ideal Gas Law is based on Boyle's Law, Gay-Lussac's Law, Charles's Law, and Avogadro's Law, and it's described by the following equation:

Where,
P is the pressure of the gas.
V is the volume of the gas.
n is the number of moles of the gas.
T is the absolute temperature of the gas (Kelvin).
R is the ideal gas constant, which is equal to:
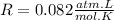
So, we are given the information:
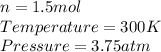
Now, substituting the given information and isolating the Volume from The Ideal Gas Law equation, we have:

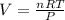
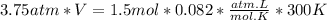
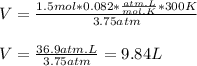
So, the approximate volume is 9.84 L.
Have a nice day!