Hello!
The answer is:
The new volume is 2.84 L.
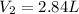
Why?
To solve the problem, we need to remember what STP means. STP means that the gas is at standard temperature and pressure, or 273.15 K (0°C) and 1 atm.
Also, we need to use the Combined Gas Law, since the temperature, the pressure and the volume are being changed.
The Combined Gas Law establishes a relationship between the temperature, the pressure and the volume of an ideal gas using , Gay-Lussac's Law, Charles's Law, and Boyle's Law.
The law is defined by the following equation:
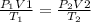
Where,
 is the first pressure.
is the first pressure.
 is the first volume.
is the first volume.
 is the first temperature.
is the first temperature.
 is the second pressure.
is the second pressure.
 is the second volume.
is the second volume.
 is the second temperature.
is the second temperature.
So, we are given the following information:
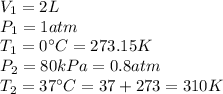
Then, isolating the new volume, and substituting, we have:
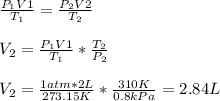
Hence, the new volume is 2.84 L.
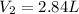
Have a nice day!