Hello!
The answer is:
The new pressure will be equal to 6 atm.
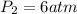
Why?
Since we know that the temperature is constant, we can use the Boyle's Law to solve the problem.
The Boyle's Law establishes that when the temperature is kept constant, the pressure and the volume will be proportional.
So, we have the equation:
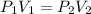
We are given,
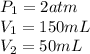
Substituting and isolating
 from the Boyle's Law, we have:
from the Boyle's Law, we have:
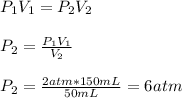
Hence, the new pressure will be equal to 6 atm.
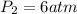
Have a nice day!