Answer:
You will need 7.953 kJ heat energy to raise the temperature of water from 277K to 315K.
Step-by-step explanation:
To calculate how much heat is needed to raise the temperature of any substance, you need:
The mass of the substance, m, which for you is 50.0 g
The temperature change that occurs, ΔT, which for you is:
Δ
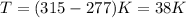
The specific heat capacity of the substance, c,
For water, the value of c is 4.186J/gK
Then, this is the equation you need:
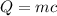 ΔT
ΔT
Where Q is the heat needed
So,
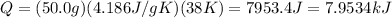
You need 7.953 kJ to raise the temperature of water from 277K to 315K.