Answer:
B) A 3-kg mass experiences a temperature rise of 50°C.
Step-by-step explanation:
As we know that the rise in temperature for 3 kg object is 50 degree C then the heat added is given as 1000 J
so here we can say that formula of heat is
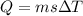
here we will have
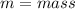
s = specific heat capacity
 = temperature rise
= temperature rise
so here if we make all three parameters same to make this heat energy same
so correct answer will be
B) A 3-kg mass experiences a temperature rise of 50°C.