For this case we have that, according to the periodic table, the atomic mass of the Carbon is
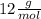 and that of the Oxygen is
and that of the Oxygen is
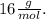
By definition, the number of moles is given by:
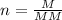
Where:
M: It's the mass
MM: It's the molar mass
n: It is the number of moles
10 milligrams equals to 0.01 grams
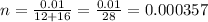
ANswer:
Option C