Hello!
The answer is: Charle's Law.
Why?
The law that states that the volume and absolute temperature of a fixed quantity of gas (ideal gas) are proportional under constant pressure is the Charle's Law, also known as the law of volumes.
The law describes how a gas kept under constant pressure tends to expand when the temperature increases and it's described by the following equation:
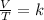
Where,
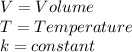
Also, to describe the relationship between two differents volumes at different temperatures, we have:
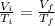
Where,

Have a nice day!