Answers:
The energy
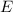 of a photon is given by the following formula:
of a photon is given by the following formula:
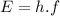 (1)
(1)
Where:
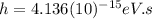 is the Planck constant
is the Planck constant
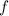 is the frequency
is the frequency
Now, the frequency has an inverse relation with the wavelength
 :
:
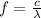 (2)
(2)
Where
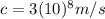 is the speed of light in vacuum
is the speed of light in vacuum
Substituting (2) in (1):
 (3)
(3)
Knowing this, let's begin with the answers:
(a) Microwave: 50.00 cm
For
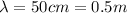
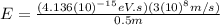
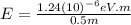
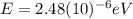
(b) Visible: 500 nm
For
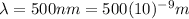
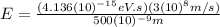
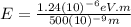
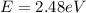
(c) X-ray: 0.5 nm
For
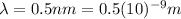
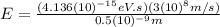
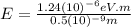
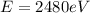
As we can see, as the wavelength decreases, the energy increases.