A) 350 J
- The initial internal energy of the cup is
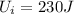
- The final internal energy of the cup is
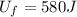
According to the first law of thermodynamics:
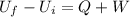
where
Q is the heat absorbed by the system
W is the work done on the system
The work done on the system in this case is 0, so we can rewrite the equation as
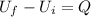
And so we find the heat transferred
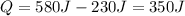
B) IN the cup
Step-by-step explanation:
in this situation, we see that the internal energy of the cup increases. The internal energy of an object/substance is proportional to its temperature, so it is a measure of the average kinetic energy of the molecules of the object/substance. Therefore, in this case, the temperature (and the energy of the molecules of the substance) has increased: this means that heat has been transferred INTO the system from the environment (the heat came from the sun).