Hello!
The answer is:
4 moles of
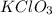 are required to produce 6 moles of
are required to produce 6 moles of

Why?
To solve stoichiometric problems, we need to write the balanced equation of the compound that we are working with.
Potassium chlorate decomposition is given by the following equation:
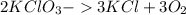
Now, from the equation we know that 3 moles of
 gives 2 moles of
gives 2 moles of
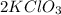 , we can calculate how many moles of the same compound react to produce 6 moles of
, we can calculate how many moles of the same compound react to produce 6 moles of
 the following equation:
the following equation:
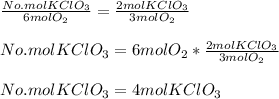
Hence, we have that 4 moles of
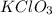 are required to produce 6 moles of
are required to produce 6 moles of

Have a nice day!