Answer: Option (a) is the correct answer.
Step-by-step explanation:
A single replacement reaction is defined as the reaction in which a single element displaces another element from a compound.
For example,
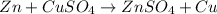
Only a more reactive element can replaces another element from a compound.
So, according to reactivity series zinc element is more reactive than copper. Hence, zinc is able to displace copper in a copper sulfate solution.
Thus, we can conclude that the type of reaction you expect for the reactants Zn and
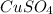 is single replacement.
is single replacement.