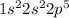Answer: Option (b) is the correct answer.
Step-by-step explanation:
As the atomic number of fluorine is 9. This means that there are 9 protons present in an atom of fluorine.
This is because atomic number is the number sum of total nmber of protons present in an atom.
As in a neutral atom, the number of protons is equal to the number of electrons. Since,there are 9 electrons present in given fluorine atom.
Hence, electronic configuration of this fluorine atom is as follows.