Answer:
 gas diffuses faster than
gas diffuses faster than

Step-by-step explanation:
Diffusion of a gas is defined as the amount of volume of gas displaced in a given amount of time. The diffusion of gas is determined by using Graham's Law.
This law states that the rate of diffusion of gas is inversely proportional to the square root of its molar mass. The equation given by this law follows:
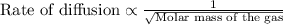
More the molar mass of the gas, its rate of effusion will be less and vice-versa.
We are given:
Molar mass of carbon dioxide = 44 g/mol
Molar mass of propane = 44.10 g/mol
As, the molar mass of carbon dioxide is less than the molar mass of propane by 0.1 unit, so rate of diffusion of carbon dioxide is faster than the rate of effusion of propane.
Hence,
 gas diffuses faster than
gas diffuses faster than
