Answer:
Na+
Step-by-step explanation:
Spectator ions are ions that remain unchanged in a chemical reaction i.e. they exists in the same form in the reactant and the product side of the equation. Since these ions do not actually participate in the chemical reaction, they are termed as spectator ions.
The reaction between HCl and NaOH is a neutralization reaction yielding salt NaCl and water H2O.
HCl and NaOH are strong acids and bases respectively which completely dissociate into ions. NaCl is also an ionic compound which exists as Na+ and Cl-. The chemical reaction is:
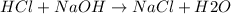
The total ionic equation is:
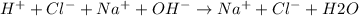
Based on the above equation: Na+ and Cl- ions remain the same on the reactant and product side. Hence, they are the spectator ions.