Answer: The correct answer is Beryllium.
Step-by-step explanation:
Ionization energy is defined as the energy required to remove valence electron from an isolated gaseous atom. It is expressed as
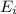
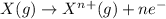
Energy required for a stable molecule (having fully filled and half filled electronic configuration)
The electronic configuration for the elements given are:
1. Be :
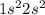
This element will have the highest ionization energy because this element has stable electronic configuration and electrons present in '2s' orbital is near to the nucleus. So, to remove this electron, we need a huge amount of energy.
2. B :
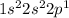
This element does not have stable electronic configuration. So, this will not have highest ionization energy.
3. Ne :
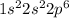
As, this element has stable electronic configuration but the valence electron is far from the nucleus and does not require a huge amount of energy to remove it. So, this will not have highest ionization energy.
4. O :
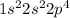
This element does not have stable electronic configuration. So, this will not have highest ionization energy.
From the above information, we can conclude that the Beryllium has the highest ionization energy.