Hello!
If all the water in 430.0 mL of a 0.45 M NaCI solution evaporates what is the mass of NaCI will remain
We have the following data:
M (Molarity) = 0.45 M (or 0.45 mol/L)
m1 (mass of the solute) = ? (in grams)
V (solution volume) = 430.0 mL → 0.43 L
MM (molar mass of NaCl) = 23u + 35.44u = 58.44u (or 58.44 g/mol)
Now, let's apply the data to the formula of Molarity, let's see:
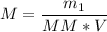
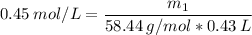
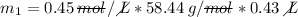

__________________________
Answer:
The mass of NaCl is approximately 11.31 grams
_______________________