Answer:
Li₂O
Step-by-step explanation:
To calculate the empirical formula first you need to convert your given into moles. You can do this by first finding how many grams there are of each substance in 1 mole, which is given by their atomic mass.
lithium = 6.94 g/mole
oxygen = 16.00 g/mole
We use this then to convert our given into moles:

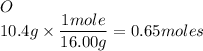
Then we take the number of moles and divide it by the smallest calculated amount and round it of to the nearest WHOLE number.
In this case the smallest calculated amount is 0.65moles, so we use this to divide.
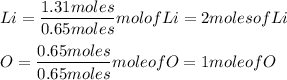
The molar ratio of Li to O is 2:1
The empirical formula is then:
Li₂O₁ or simply Li₂O