Answer: The correct answer is Option D.
Step-by-step explanation:
Endothermic reactions are defined as the reactions in which energy is absorbed by the system. The potential energy of the products is greater than the potential energy of the reactants. The total enthalpy change of the reaction,
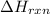 comes out to be positive.
comes out to be positive.
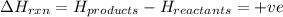
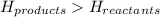 for endothermic reactions
for endothermic reactions
Exothermic reactions are defined as the reactions in which energy is released by the system. The potential energy of the products is less than the potential energy of the reactants. The total enthalpy change of the reaction,
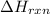 comes out to be negative.
comes out to be negative.
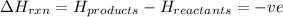
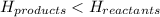 for exothermic reactions
for exothermic reactions
Hence, the correct answer is Option D.