Hello!
The answer is:
There are 732.24 grams of Dinitrogen Pentoxide
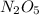 in 6.78 moles of the same compound.
in 6.78 moles of the same compound.
Why?
The dinitrogen pentoxide, or nitrogen pentoxide, is a rare salt that consists of anion and cations, and it's an important compound when preparing some kinds of explosives.
So, to answer the question, first, need to look for the molecular formula of the dinitrogen pentoxide, then, use it to calculate the mass in gram of 6.78 moles of the same compound.
The dinitrogen pentoxide formula is:
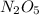
Where, the molar mass of each element are:
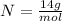
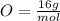
Calculating the molar mass of the compound, we have:
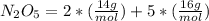
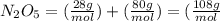
Now, calculating what is the mass of 6.78 of
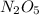 , we have:
, we have:
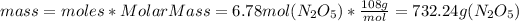
So, there are 732.24 grams of Dinitrogen Pentoxide
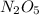 in 6.78 moles of the same compound.
in 6.78 moles of the same compound.
Have a nice day!