Answer: 2.56 moles
Step-by-step explanation:
To calculate the moles :
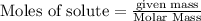
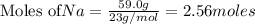
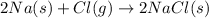
Na is the limiting reagent as it limits the formation of product and chlorine is the excess reagent.
According to stoichiometry :
2 moles of Na produce = 2 moles of NaCl
Thus 2.56 moles of Na produce =
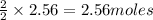 of NaCl
of NaCl
Thus 2.56 moles of NaCl are produced.