Answer: (a)
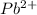
Explanation:
The metal with negative reduction potential will easily lose electrons and thus is oxidized and the one with positive reduction potential will easily gain electrons and thus is reduced.
Where both
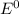 are standard reduction potentials.
are standard reduction potentials.
![E^0_([Pb^(2+)/Pb])=-0.126V](https://img.qammunity.org/2020/formulas/chemistry/high-school/7wr853akmm08r3z08ec00or7nus199ibc7.png)
![E^0_([Cr^(3+)/Cr])=-0.74V](https://img.qammunity.org/2020/formulas/chemistry/high-school/wfijs5tbn0yd5877ci54nvobrg4ufb9x5r.png)
![E^0_([Fe^(2+)/Fe])=-0.44V](https://img.qammunity.org/2020/formulas/chemistry/high-school/du5w62c5r1m4u7x1g3r3j8jyzq1f7kyvgh.png)
![E^0_([Sn^(2+)/Sn])=-0.13V](https://img.qammunity.org/2020/formulas/chemistry/high-school/gkbtr2oafr3eeyc2ta6t3ziwn816vxselx.png)
Thus here
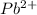 with negative reduction potential and least magnitude has the most tendency to gain electrons and thus can be most easily reduced.
with negative reduction potential and least magnitude has the most tendency to gain electrons and thus can be most easily reduced.