Step-by-step explanation:
A chemical reaction is defined as the reaction that involves exchange of electrons between the reacting atoms.
For example,
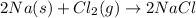
Mass of Na = 23 g/mol
Mass of Cl = 35 g/mol
Mass of NaCl compound = (23 + 35) g/mol = 58 g/mol
So, in chemical reactions law of conservation of mass is obeyed because mass of reacting species is changing from one form to another.
Whereas in nuclear reactions there is splitting or combining of atomic nuclei of atom(s). Since, nuclei is involved in nuclear reaction so more energy is required.
Also, E =
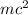 so, in nuclear reactions mass of reacting atoms changes into energy. This results in formation of larger amount of energy.
so, in nuclear reactions mass of reacting atoms changes into energy. This results in formation of larger amount of energy.
Therefore, we can conclude that in situations involving equal masses, chemical reactions produce less energy than nuclear reactions.