Answer: Option A
About 0.02 pounds
Explanation:
We know that density is defined as:
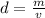
Where
d is the density
m is the mass
v is the volume
We want to calculate the helium-air mass of a spherical balloon and we know its density and the diameter of the balloon.
Then we can calculate its mass.
1) We use the diameter of the spherical globe to calculate its volume.
The volume of a sphere is:
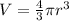
and
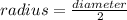
So
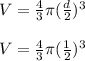
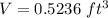
2) Use the density formula to find the mass
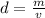
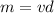
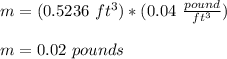
The answer is 0.02 pounds