Answer : The incorrect statement is, Lewis acid is a substance that donates a lone-pair of electrons.
Explanation :
According to the Arrhenius theory, an acid is a substance that ionizes in the water to give hydronium ion
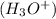 or hydrogen ion
or hydrogen ion
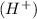 and a bases is a substance that ionizes in the water to give hydroxide ion
and a bases is a substance that ionizes in the water to give hydroxide ion
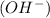 .
.
According to the Bronsted Lowry concept, Bronsted Lowry-acid is a substance that donates one or more hydrogen ion in a reaction and Bronsted Lowry-base is a substance that accepts one or more hydrogen ion in a reaction.
According to the Lewis acid-base theory, an acid is a substance that accepts pairs of electrons and the a base is a substance that donates pairs of electrons.
The pH of an acid is always less than 7.
Hence, the incorrect statement is, Lewis acid is a substance that donates a lone-pair of electrons.