The given question is an incomplete question, the complete question is given below.
Use this equation to answer the questions that follow.
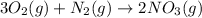
5 moles of oxygen gas are present. How many moles of
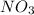 will be produced with this amount of oxygen gas?
will be produced with this amount of oxygen gas?
Answer : The moles of
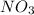 produced will be 3.33 moles.
produced will be 3.33 moles.
Explanation : Given,
Moles of oxygen gas = 5 moles
Now we have to calculate the moles of
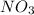 .
.
The given balanced chemical equation is:
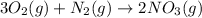
From the balanced chemical equation we conclude that,
As, 3 moles of
 gas react to produces 2 moles of
gas react to produces 2 moles of
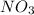 gas
gas
So, 5 moles of
 gas react to produces
gas react to produces
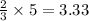 moles of
moles of
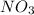 gas
gas
Therefore, the moles of
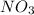 produced will be 3.33 moles.
produced will be 3.33 moles.