Answer:
Epsom salt is Magnesium sulphate
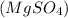
So the chemical reaction is
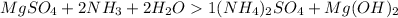
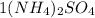 contains
contains
 mole of H atoms
mole of H atoms
That is to find the number of atoms we multiply coefficient subscript number outside the Bracket
1 mole of H atoms contains
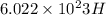 atoms
atoms
So,
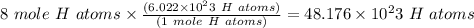
Moving the decimal point to the right, the power of 10 decreases
So, we get
=
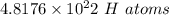
=
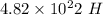 atoms is the Answer.
atoms is the Answer.