Answer: 1.07 V
Explanation:
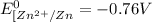
![E^0_([Cu^(2+)/Cu])=+0.34V](https://img.qammunity.org/2020/formulas/chemistry/high-school/jdqrwlz4jxg7h52em0knt32e6msp300q9m.png)
The metal with negative reduction potential will easily lose electrons and thus is oxidized and the one with positive reduction potential will easily gain electrons and thus is reduced.
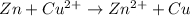
 = standard electrode potential =
= standard electrode potential =
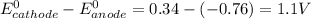
Using Nernst equation:
![E_(cell)=E^o_(cell)-(0.0592)/(n)\log ([Zn^(2+)])/([Cu^(2+)])](https://img.qammunity.org/2020/formulas/chemistry/high-school/g8vtuakc2eabagjfd8pkgvunltzg6xbi7a.png)
where,
n = number of electrons in oxidation-reduction reaction = 2
![E_(cell)=1.10-(0.0592)/(2)\log ([0.1])/([0.01])](https://img.qammunity.org/2020/formulas/chemistry/high-school/chd278l50fqgiec1a9jvwi7imsv0ent5gs.png)
