Step-by-step explanation:
When a bond between two atoms is formed by transfer of electrons then the bond is known as ionic bond. On the other hand, when a bond is formed between two atoms by sharing of electrons then the bond is known as covalent bond.
For example, carbon atom needs 4 more electrons in order to complete its octet. Whereas hydrogen needs one more electron to become stable. Hence, carbon will share its valence electrons with hydrogen atoms and therefore carbon will combine with 4 hydrogen atoms.
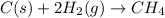
Thus, we can conclude that two atoms are bonded through the unequal sharing of electrons then covalent bond exists between the atoms.