Answer: as mass increases, the wave nature of matter is less easy to observe.
At the beginning of the 20th century the French physicist Louis De Broglie proposed the existence of matter waves, that is to say that all matter has a wave associated with it.
In this sense, the de Broglie wavelength
 is given by the following formula:
is given by the following formula:
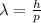 (1)
(1)
Where:
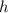 is the Planck constant
is the Planck constant
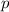 is the momentum of the atom, which is given by:
is the momentum of the atom, which is given by:
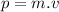 (2)
(2)
Where:
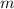 is the mass
is the mass
 is the velocity
is the velocity
Substituting (2) in (1):
![\lambda=(h)/(m.v)[\tex] <strong> (3)</strong></p><p></p><p>As we can see, <strong>if we increase the mass, the wavelength decreases </strong>(because [tex]\lambda](https://img.qammunity.org/2020/formulas/physics/high-school/7njj5au3wb87rx6d1pp1p2wgi97b6qp8je.png) is inversely proportional to
is inversely proportional to
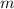 ).
).
Therefore, if the wavelength decreases the wave nature of matter is less easy to observe.
The other options are incorrect because:
a) as
 increases
increases
 decreases and the particle nature matter becomes more evident
decreases and the particle nature matter becomes more evident
b) as
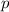 decreases
decreases
 increases and the wave nature matter becomes more evident
increases and the wave nature matter becomes more evident
c) There is also a relation between the wavelength and the energy
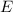 :
:
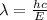
So, as energy increases, the particle nature matter becomes more evident and the wave nature of matter becomes harder to observe