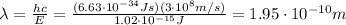Answer:
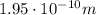
Step-by-step explanation:
First of all, we need to calculate the energy of the x-ray photon emitted during the transition from K-shell to L-shell, and this energy is equal to the difference in energy between the two levels:
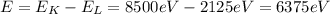
Converting into Joules,
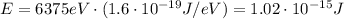
Now we know that the energy of the photon is related to its wavelength by:

where
h is the Planck constant
c is the speed of light
 is the wavelength
is the wavelength
Re-arranging the equation for
 , we find
, we find