Answer:
4186 Joules
Step-by-step explanation:
The specific heat capacity of a substance is defined as the amount of heat needed to raise the temperature of 1 kg of the substance by 1 Kelvin. In formula,
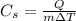
where
Q is the amiunt of heat needed
m = 1 kg is the mass
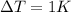
is the variation of temperature of the substance
For water, the specific heat capacity is
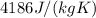 . This means that the heat energy required to raise 1 kg of water by 1 K is exactly 4186 J.
. This means that the heat energy required to raise 1 kg of water by 1 K is exactly 4186 J.