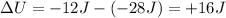Answer:
+16 J
Step-by-step explanation:
We can solve the problem by using the 1st law of thermodynamics:
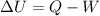
where
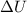 is the change of the internal energy of the system
is the change of the internal energy of the system
Q is the heat (positive if supplied to the system, negative if dissipated by the system)
W is the work done (positive if done by the system, negative if done by the surroundings on the system)
In this case we have:
Q = -12 J is the heat dissipated by the system
W = -28 J is the work done ON the system
Substituting into the equation, we find the change in internal energy of the system: