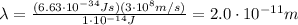1. Single dental x-rays:
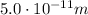
The energy of the photon is
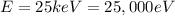
Using the conversion factor
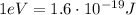
we can convert it into Joules:
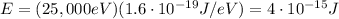
The relationship between photon energy and wavelength is
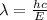
where
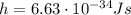 is the Planck constant
is the Planck constant
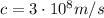 is the speed of light
is the speed of light
E is the energy
Substituting into the formula, we find
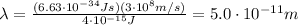
2. Microtomography:
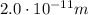
The energy of these photons is 2.5 times greater, so
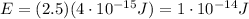
And by applying the same formula used at point 1, we find the corresponding wavelength: