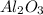Answer: The correct answer is
Step-by-step explanation:
Aluminium is the 13th element of the periodic table with electronic configuration of
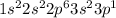
This element looses 3 electrons and shows an oxidation state of +3.
Oxygen is the 8th element of the periodic table with electronic configuration
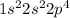
This element will gain 2 electrons and shows an oxidation state of -2.
By criss-cross method, the oxidation state of aluminium and oxygen gets revered and they form the coefficients of the compound.
Hence, the compound formed will have the chemical formula of