Answer: Option (A) is the correct answer.
Step-by-step explanation:
Atomic mass is defined as the summation of total number of protons and neutrons present in an element.
For example, in
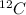 the number 12 represents the total number of protons and neutrons present in it.
the number 12 represents the total number of protons and neutrons present in it.
As it is known that an atom of carbon always contains 6 protons but it can hold different number of neutrons.
Hence, in
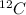 the number 12 represents the atomic mass.
the number 12 represents the atomic mass.
Therefore, we can conclude that the number to the right of an element's symbol (ex. C-12) identifies the atomic mass.