Answer:
mass of silver chloride (AgCl) = 3.0135 grams
Step-by-step explanation:
The chemical equation is:
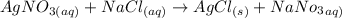
No. of mole = molarity × volume
For AgNO3:
No. of moles = 1.00 × 0.055
= 0.055 moles
No. of moles of NaCl = 0.84 × 0.025
= 0.021 moles
From the equation:
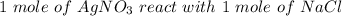
Therefore, 0.055 moles of AgNO3 react with 0.055 moles of NaCl
Here;
NaCl serves as the limiting reactant
Thus,
1 mole of NaCl will react with AgNO3 to yield 1 mole of AgCl
∴
0.021 moles of NaCl react with AgNO3 to yield 0.021 moles og AgCl
mass of AgCl = no of moles × molar mass
= 0.021 × 143.5
= 3.0135 grams