Hello!
If a solution containing 75 mL of alcohol has a percent by volume of 50%. What is the total volume of the solution ?
We have the following information:
% v/v (percentage of volume per volume) = 50
V1 (solute volume) = 75 mL
V (volume of the solution) = ? (in mL)
We apply the data to the formula of volume percentage of the solute per volume of solution, we will have:
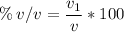
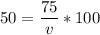
multiply the means by the extremes
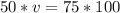
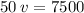
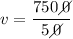
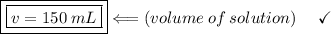
________________________