Answer:
e. 4.847 x 10-19 J
Step-by-step explanation:
From the given information:
The equation connecting the photon energy and the wavelength is:
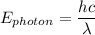
where;
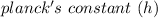 = 6.626 * 10 ^{-34} J.s
= 6.626 * 10 ^{-34} J.s
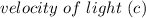 = 3.00 * 10^8 m/s
= 3.00 * 10^8 m/s
wavelength
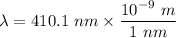
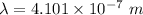
To determine the photon energy of violet light
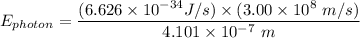
= 4.847 × 10⁻¹⁹ J