Answer:
The equilibrium constant for the system at this temperature is 15.4074.
Step-by-step explanation:
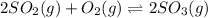
Concentration of
![[SO_2]](https://img.qammunity.org/2020/formulas/chemistry/high-school/iragxlotv7fynhtzk51bo047gouj0vdgvk.png) = 0.344 mol/L
= 0.344 mol/L
Concentration of
![[O_2]](https://img.qammunity.org/2020/formulas/chemistry/high-school/ngke6m6k9deep00fr55hbg5jnvxrxzrnj0.png) = 0.172 mol/L
= 0.172 mol/L
Concentration of
![[SO_3]](https://img.qammunity.org/2020/formulas/chemistry/high-school/cf56dwobaje5ymnwzisu2eafq5mi45yoj9.png) = 0.56 mol/L
= 0.56 mol/L
The equilibrium constant of the given equilibrium equation will be given as:
![k_(eq)=([SO_3]^2)/([SO_2]^2[O_2])](https://img.qammunity.org/2020/formulas/chemistry/high-school/xol50nepuracz0lceavk7nm3qnqrur1sma.png)
![k_(eq)=([0.56 mol/L]^2)/([0.344 mol/L]^2[0.172 mol/L])=15.4074](https://img.qammunity.org/2020/formulas/chemistry/high-school/eftt2q4ov3t52jmmlm3krojdj61ngnm3u0.png)
The equilibrium constant for the system at this temperature is 15.4074.