Answer:
1,075.5 kiloJoules of heat released for the combustion of 0.500 moles of propane.
Step-by-step explanation:
Heat released on combustion of 88.0 grams of propane = 4,032 kJ
Moles of propane =
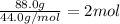
Heat released on combustion of 2 moles of propane = 4,032 kJ
Heat released on combustion of 0.500 moles of propane :
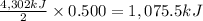
1,075.5 kiloJoules of heat released for the combustion of 0.500 moles of propane.