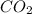Answer: A. 20.0L
Step-by-step explanation:
According to avogadro's law, equal moles of all gases occupy equal volumes at STP.
The given balanced equation is:
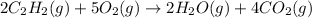
According to stoichiometry :
4 L of
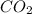 is formed by = 2 L of
is formed by = 2 L of
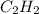
Thus 40.0 L of
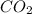 is formed by =
is formed by =
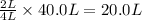 of
of
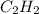
Thus 20.0 L of
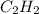 is required to form 40.0L of
is required to form 40.0L of