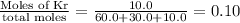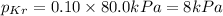Answer: The partial pressure of Argon , Neon and krypton are 48 kPa , 24 kPa and 8 kPa respectively.
Step-by-step explanation:
According to Raoult's Law , the partial pressure of each component in the solution is equal to the total pressure multiplied by its mole fraction. It is mathematically expressed as
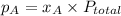
where,
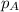 = partial pressure of component A
= partial pressure of component A
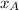 = mole fraction of A
= mole fraction of A
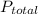 = total pressure
= total pressure
mole fraction of Ar =
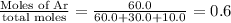
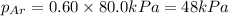
mole fraction of Ne =
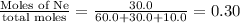
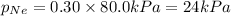
mole fraction of Kr =