Answer: 3.61 L
Step-by-step explanation:
To calculate the moles, we use the equation:
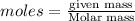
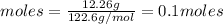
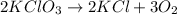
2 moles of
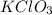 produce = 3 moles of
produce = 3 moles of

0.1 moles of
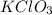 produce =
produce =
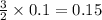 moles of
moles of

According to the ideal gas equation:'

P = Pressure of the gas = 1 atm (NTP)
V= Volume of the gas = ?
T= Temperature of the gas = 20°C = (20+273) K = 293 K (NTP)
R= Value of gas constant in in kilopascals = 0.0821 Latm/K mol

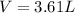
Thus volume of oxygen at NTP obtained by decomposing 12.26 g of
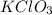 is 3.61 L
is 3.61 L